FEATURED POST
What You Can Learn from 7 Theme Fusion Success Stories
Nam lacinia arcu tortor, nec luctus nibh dignissim eu. Nulla sit amet maximus nulla. Pellentesque a accumsan eros, ac molestie nulla. Morbi interdum in neque vitae vulputate.
Donato Bonifazi, CVBF’s CEO to Represent TEDDY, the European Network of Excellence for Paediatric Research, in the ACT EU Multi-Stakeholder Platform Annual Meeting
We are pleased to announce that our CEO, Donato Bonifazi, a permanent member of the Advisory Group of the ACT EU Multistakeholder [...]
Giovanni Migliaccio: Advancing Personalised Medicine for Rare Diseases
In the realm of rare disease research and education, collaboration and knowledge sharing are paramount. Giovanni Migliaccio, the CVBF’s Scientific Director [...]
Our CEO to Present at Prestigious Global CardioVascular Clinical Trialists Workshop
We are proud to announce that Donato Bonifazi, CVBF’s CEO and Member of the Board of Directors of the Teddy European Network [...]
EPTRI’s 2024 Scientific Meeting
The European Paediatric Translational Research Infrastructure (EPTRI) has exciting news to share about its upcoming Scientific Meeting and legal [...]
iCAN 2024 Advocacy and Research Summit
Mark your calendars! The 2024 iCAN Advocacy and Research Summit is scheduled to take place from 15-19 July in Bari, [...]
CVBF’s Researchers Publications in Paediatric Data Standardization
Recent publications from researchers affiliated with the CVBF consortium underscore our commitment to advancing efforts in paediatric data standardization. The [...]
DEEP-2 Lay Summaries for Children and Teens
Thanks to the collaboration with the TEDDY Network, CVBF has published two new lay summaries explaining the key findings [...]
Newsletter, December 2023
Newsletter, December 2023 Starting the new year in a larger, more accessible office at Viale Cesare Battisti 17, Pavia (IT), CVBF embodies growth in [...]
CVBF’s CEO selected as Permanent Member of the Advisory Group of the ACT EU Multistakeholder Platform (MSP AG)
On 20 December 2023, Donato Bonifazi, our CEO, has been selected as a permanent member of the Advisory Group of the ACT EU Multistakeholder [...]
CVBF Wins ERAMET Project, Funded by Horizon Europe
CVBF has achieved a major milestone by securing a significant role in the Horizon Europe-funded ERAMET project. Guided by Prof Adriana Ceci, the Lead Scientist [...]
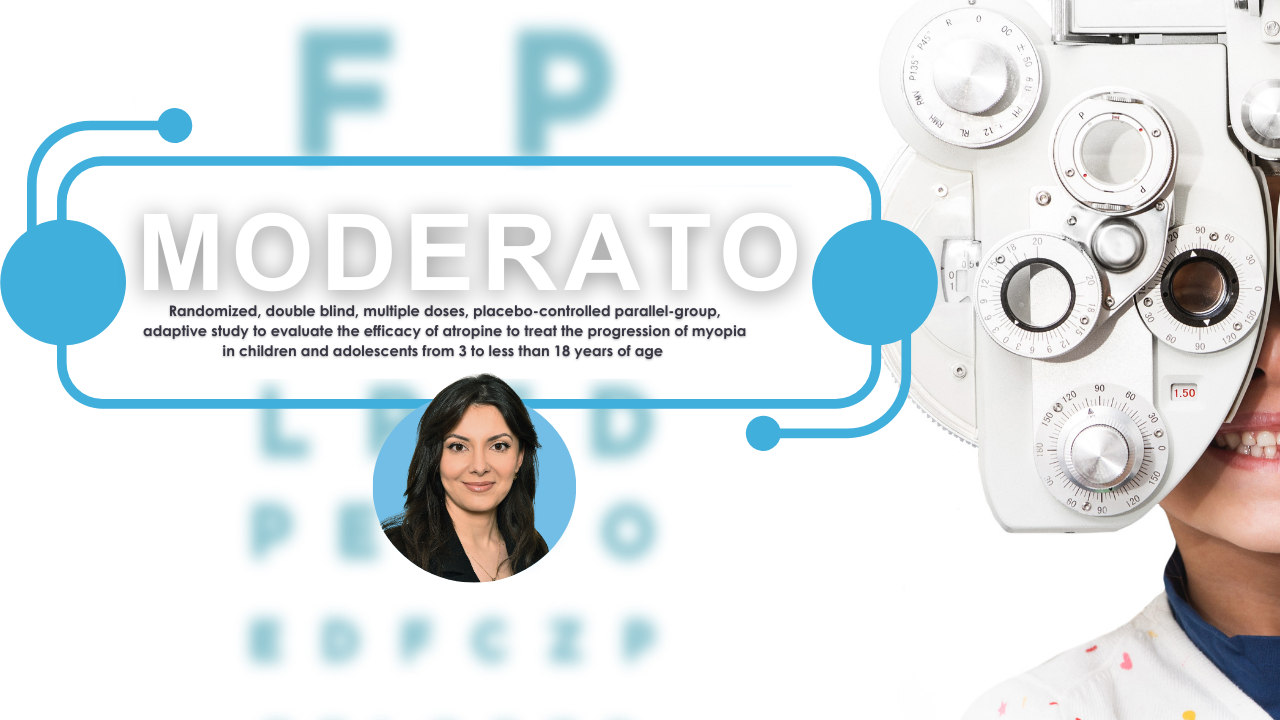
New Clinical Trial Seeks to Slow Progression of Nearsightedness in Children
A new international clinical trial is underway across Europe to test whether low-dose atropine eye drops can effectively slow the progression of myopia (nearsightedness) in [...]
13 Organizations Across Europe Join Forces for Novel ATMP on Faecal incontinence.
CVBF provides regulatory, site management, and monitoring support for the AMELIE (Anchored Muscle cELls for IncontinencE) project, Ana Dilo as our Clinical Project Manager of [...]
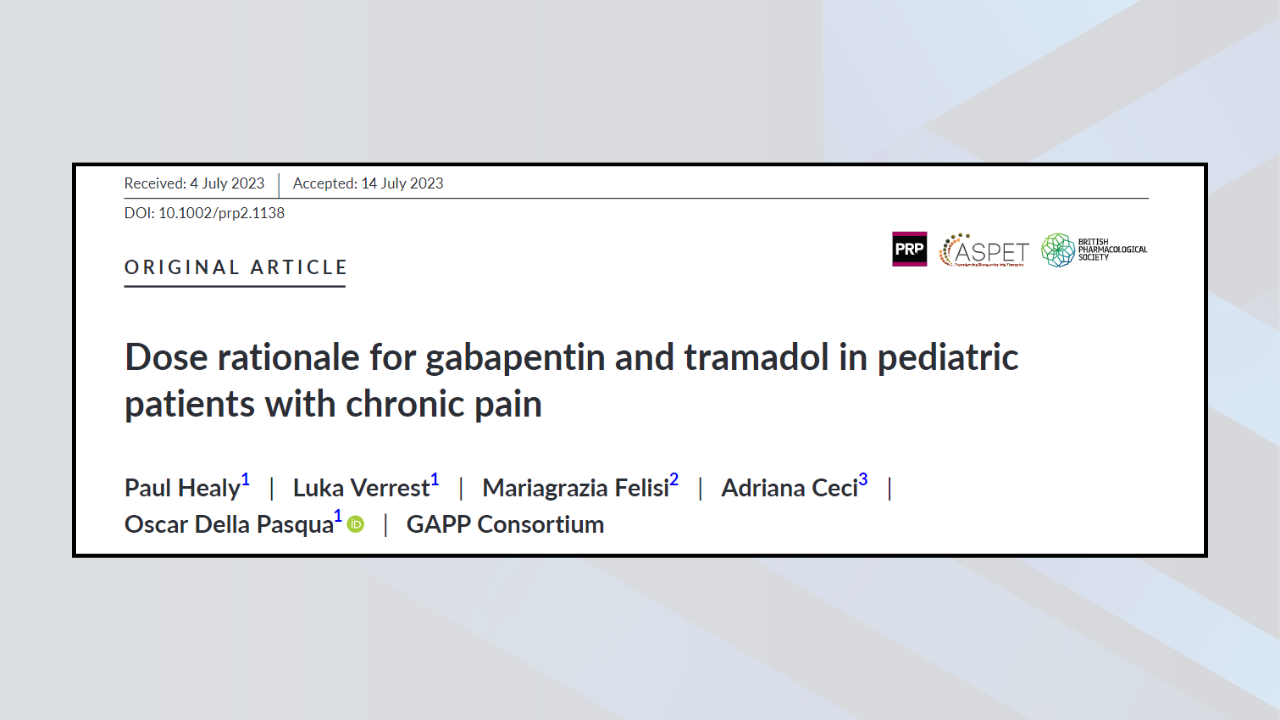
New Article on Gabapentin and Tramadol in Paediatric Patients with Chronic Pain
A new article published on 6 October 2023 in the Journal of the British Pharmacological Society titled"Dose rationale for gabapentin and tramadol in paediatric patients [...]