We are very pleased to announce that the ID-EPTRI (European Paediatric Translational Research Infrastructure) project, coordinated by CVBF and submitted within the INFRADEV-2016-2017 single-stage call for proposals, aimed to create the framework for a new paediatric Research Infrastructure (RI) has been successfully evaluated and granted 3.00 million Euro in funding from the European Commission.
The project arises from the need to cover the gap in paediatric medicines by enhancing innovative technologies and reconnecting all the medicine research phases from discovery and preclinical to clinical research and care.
The project will develop a new RI, EPTRI, that is complementary to the existing Biomed Research Infrastructures acting as a ‘Paediatric Common Service’ in the ESFRI Scenario. The project involves 26 partners (listed below) from EU and non-EU countries including consolidated RIs, top-level universities, scientific and clinical centres of excellence in Europe and is aimed to create a Conceptual Design Report (CDR) to realize the European paediatric RI.
EPTRI will be a “one-stop-shop” for advice in paediatric drug development with demonstrated capacity to bridge the existing gaps in paediatric medicines research by connecting the different steps in research from early stages, to enable and prepare researchers in many methodological areas to conduct research that effectively underpins the development of medicines in children, to increase the global competitiveness of the European RIs and the paediatric research also in favour of children, young people and their families.
In order to set up the new RI into the European Landscape, the activities to be performed will include 3 different phases: 1) a context analysis, aimed to acquire the information needed to complete a consistent CDR; 2) an Operational phase focused more specifically on the design of the whole RI; 3) a Feasibility phase in which selected pilot experiences will allow to test a limited number of services and tools delivered by EPTRI.
The work plan of this proposal is based on 11 Work Packages:
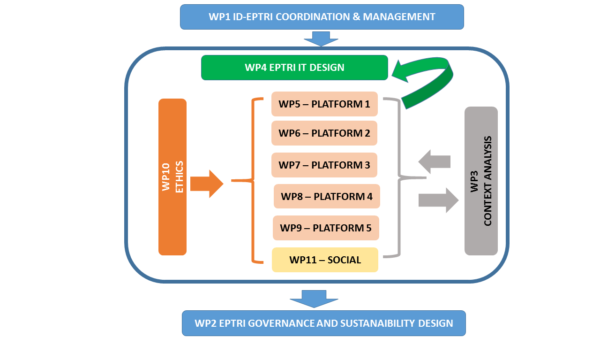
- WP1 – Coordination and Project Management. WP1 will provide the organisational framework to conduct all the activities and will continue to work until the end of the project with the day-by-day activities;
- WP2 – Governance and Sustainability. WP2 will be in charge to provide at least two possible alternative designs of the future RI;
- WP3 – Context Analysis. This is the key WP to conduct the First Phase of the project i.e. Context Analysis in order to support the Second and Third phases (Operational and Feasibility phases);
- WP4 – Concept Design, IT Structure and feasibility assessment of the Infrastructure. WP4 will be in charge to work on the RI design, the integration of the thematic platforms, the definition of the IT technologies and the set-up of pilot IT tools if needed for pilot studies;
- WP5 – Thematic Platform supporting Paediatric Medicines Discovery and Early Drug Development. This platform is aimed to implement enabling technologies in order to accelerate discovery and early development in paediatrics such as the use of bioinformatics and computer modelling, the accelerated target identification and the proof of concept studies, and the imaging techniques in System Biology;
- WP6 – Thematic Platform supporting Biomarkers Use in Paediatric Medicines Development. This platform is aimed to generate optimal conditions for the development and use of paediatric biomarkers, with particular emphasis on enhancing the potential of -omics science in paediatrics;
- WP7 – Thematic Platform on Paediatric Pharmacology. This multidisciplinary platform on human development and in vivo/in vitro modelling will support cross-disciplinary research into paediatric pharmacology implementing age appropriated technologies in drug development including animal and cellular models for paediatric diseases, human developmental pharmacology, physiologically based PK (PBPK), pop-PK and PK/PD and modelling;
- WP8 – Thematic Platform on Formulation Science. This Platform will federate the necessary expertise and competences within pharmaceutical sciences to support drug development for the paediatric population and to facilitate drug evaluation in children by providing appropriate age related formulation;
- WP9 – Thematic Platform to relate work that underpins Medicines Development to Paediatric Clinical Studies. This platform will have a huge relevance in promoting the integration of innovative technologies in paediatric drug development with clinical trials;
- WP10 – Ethics. W10 will collaborate with all the other WPs by providing advice on the context of the project documents under an ethical point of view;
- WP11 – Communication, Networking and Patients Involvement. WP11 will be aimed to develop a strategy and the tools for communication and dissemination in order to support the long-term goals of EPTRI and at the same time to contribute to generate a patient centric approach to the design of the infrastructure for the paediatric clinical research.
EPTRI Partners
(1) Consorzio per Valutazioni Biologiche e Farmacologiche (CVBF), Italy; (2) Fondazione PENTA – For the treatment and care of children with HIV – ONLUS (PENTA), Italy; (3) European Infrastructure for Translational Medicine (EATRIS ERIC), The Netherlands; (4) University College London (UCL), United Kingdom; (5) ATHENA Research and innovation center in information communication & knowledge technologies (ATHENA), Greece; (6) Biobanks and BioMolecular resources Research Infrastructure Consortium (BBMRI-ERIC), Austria; (7) The Cyprus foundation for muscular dystrophy research (CING), Cyprus; (8) Assistance Publique – Hopitaux de Paris (AP-HP), France; (9) Stichting Katholieke Universiteit (RUMC), The Netherlands; (10) The University of Liverpool (ULIV), United Kingdom; (11) Romanian Angel Appeal Foundation (RAA), Romania; (12) Instytut Pomnik Centrum Zdrowia Dziecka (IPCZD), Poland; (13) Ospedale Pediatrico Bambino Gesù (OPBG), Italy; (14) Fyziologicky Ustav Akademie ved Ceske Republiky Verejna Vyzkumna Instituce (IPHYS), Czech Republic; (15) Fundacio Sant Joan de Deu (FSJD), Spain; (16) Nizhegorodskiy Gosudarstvenniy Universitet Im N.I. Lobachevskogo (UNN), Russia; (17) Servicio Madrileno de Salud (SERMAS-HULP), Spain; (18) European Clinical Research Infrastructure Network (ECRIN-ERIC), France; (19) Qendra Spitalore Universitare Nene Tereza Tirane (UHCT), Albania; (20) TECHNION – Israel Institute of Technology (TECHNION), Israel; (21) Tartu Ulikool (UTARTU), Estonia; (22) Universitatsklinikum Erlangen (UKER), Germany; (23) Swiss Clinical Trial Organisation Verein (SCTO), Switzerland; (24) Vastra Gotaland Lans Landsting (VGR), Sweden; (25) Universitair Medisch Centrum Utrecht (UMCU), The Netherlands; (26) State “Institute of Pediatrics Obstetrics and Gynecology National Academy of Medical Sciences of Ukraine” (UKR), Ukraine.